Negative
27Serious
Neutral
Optimistic
Positive
- Total News Sources
- 4
- Left
- 1
- Center
- 0
- Right
- 1
- Unrated
- 2
- Last Updated
- 5 days ago
- Bias Distribution
- 50% Right
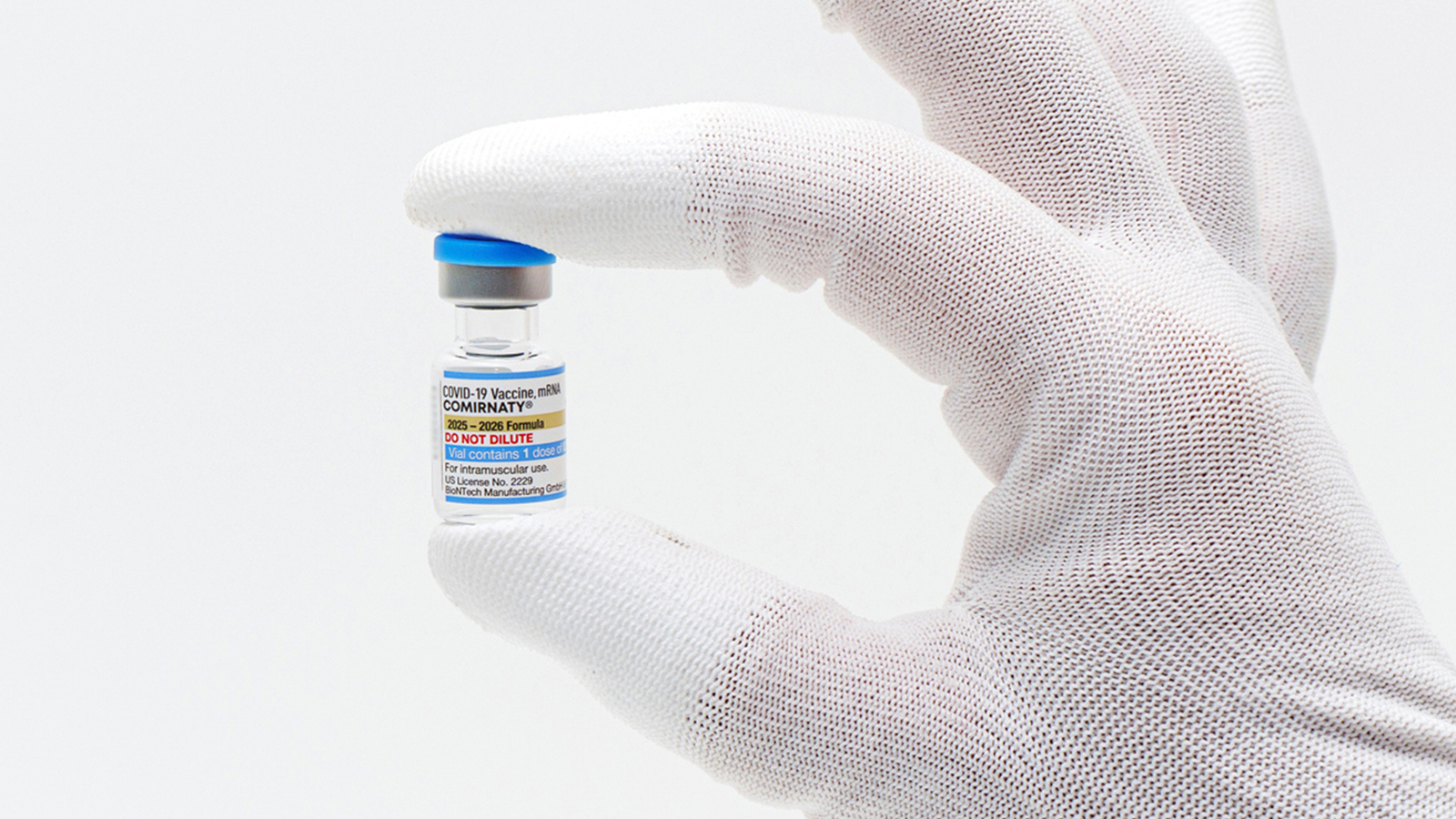

FDA Limits Updated COVID Vaccines to High-Risk Adults, Removes Pfizer Shot for Under-5s
U.S. regulators have approved updated COVID-19 vaccines from Pfizer, Moderna, and Novavax, targeting a newer variant of the virus, but with significant restrictions on eligibility. The FDA has authorized these vaccines for all seniors but limited use for younger adults and children to those with at least one high-risk health condition, such as asthma or obesity, creating new barriers for many Americans who must prove their risk to access the shots. Pfizer’s vaccine emergency authorization has been revoked for children under 5, leaving Moderna’s Spikevax as the only fully FDA-approved option for children as young as 6 months, though only for those with serious health issues. This policy marks a departure from the previous recommendation for annual vaccinations for all individuals aged 6 months and older, reflecting skepticism from Health Secretary Robert F. Kennedy Jr. and FDA Commissioner Marty Makary about the need for widespread booster shots. The decision has drawn criticism from medical groups like the American Academy of Pediatrics, which argue the limits could restrict vaccine access for families and children. Distribution and access will depend on federal, state, and private health entities, potentially delaying availability for some Americans.


- Total News Sources
- 4
- Left
- 1
- Center
- 0
- Right
- 1
- Unrated
- 2
- Last Updated
- 5 days ago
- Bias Distribution
- 50% Right
Negative
27Serious
Neutral
Optimistic
Positive
Related Topics
Stay in the know
Get the latest news, exclusive insights, and curated content delivered straight to your inbox.

Gift Subscriptions
The perfect gift for understanding
news from all angles.


