Negative
22Serious
Neutral
Optimistic
Positive
- Total News Sources
- 3
- Left
- 1
- Center
- 2
- Right
- 0
- Unrated
- 0
- Last Updated
- 18 hours ago
- Bias Distribution
- 67% Center
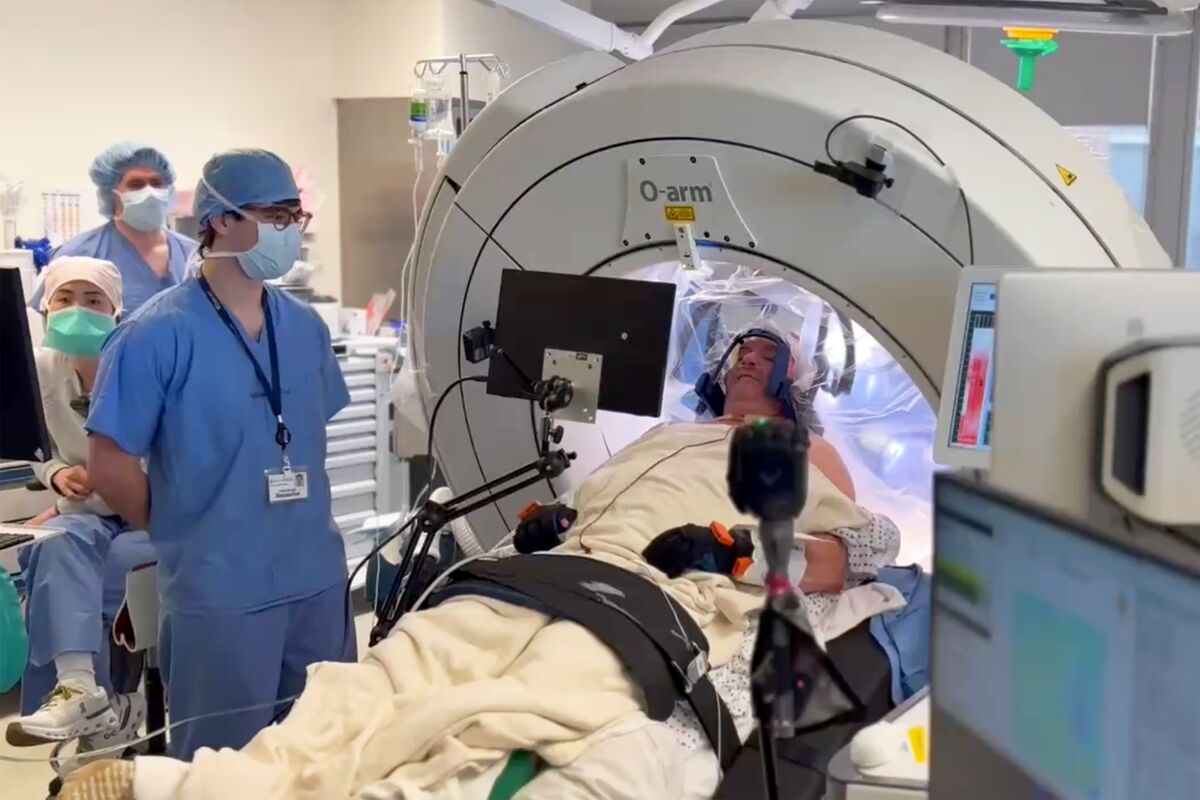

FDA Approves Precision Neuroscience's Brain Implant for Clinical Use
The U.S. Food and Drug Administration has granted regulatory clearance to a key component of Precision Neuroscience's brain-computer interface (BCI) system, the Layer 7 Cortical Interface, marking the first full approval for a wireless BCI device. This minimally invasive microelectrode array, thinner than a human hair and containing 1,024 electrodes, can be temporarily implanted for up to 30 days to decode neural signals and control external technologies. Precision aims to use the device to aid patients with severe paralysis in regaining functions such as speech and movement, while also enabling brain signal mapping during neurosurgical procedures. The device’s approval allows for clinical use and is expected to generate revenue as Precision continues to develop its broader BCI platform. Precision has already tested the Layer 7 device in 37 patients, and the FDA clearance is seen as a foundational step in expanding clinical research and improving BCI effectiveness. The company, co-founded by former Neuralink co-founder Dr. Benjamin Rapoport, now joins other leading neurotech firms such as Elon Musk’s Neuralink and Synchron in advancing brain-implant technology.



- Total News Sources
- 3
- Left
- 1
- Center
- 2
- Right
- 0
- Unrated
- 0
- Last Updated
- 18 hours ago
- Bias Distribution
- 67% Center
Negative
22Serious
Neutral
Optimistic
Positive
Related Topics
Stay in the know
Get the latest news, exclusive insights, and curated content delivered straight to your inbox.

Gift Subscriptions
The perfect gift for understanding
news from all angles.

