- Total News Sources
- 1
- Left
- 0
- Center
- 1
- Right
- 0
- Unrated
- 0
- Last Updated
- 335 days ago
- Bias Distribution
- 100% Center

NHS Resumes UK Blood Plasma Use After Ban
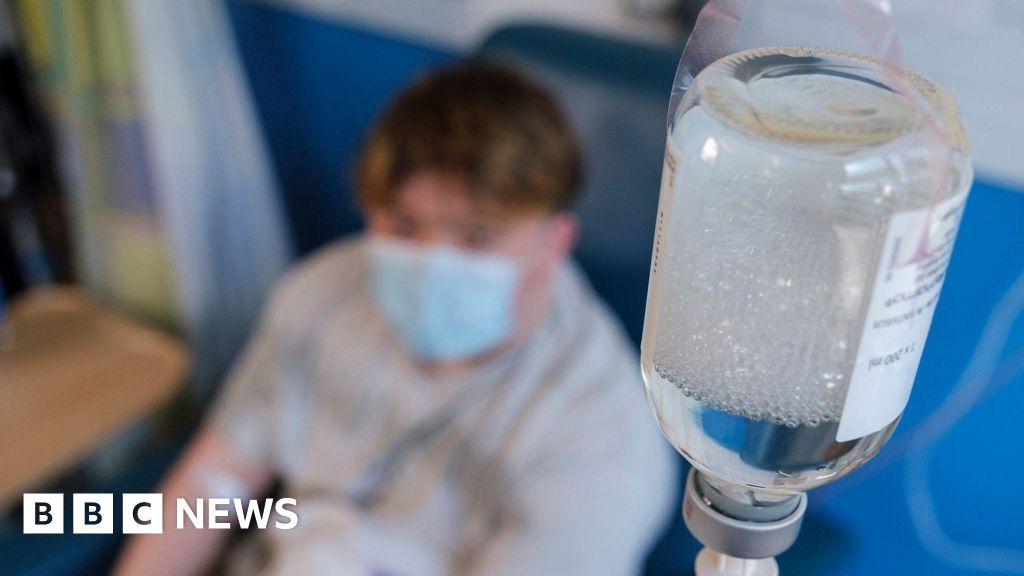
The NHS has resumed using UK-sourced blood plasma for the first time since a ban was imposed in 1988 due to concerns over variant Creutzfeldt-Jakob Disease (vCJD) linked to mad cow disease. This move aims to reduce reliance on imported plasma-derived medicines, saving the NHS between £5 million to £10 million annually and enhancing healthcare security. Since lifting the ban, the NHS has collected 250,000 liters of plasma from English donors and begun treating patients with conditions such as immunodeficiencies and autoimmune diseases. The NHS plans to supply 25% of its immunoglobulin needs domestically by the end of the year, with the goal to increase this to 30-35% by 2031 and provide 80% of albumin by 2026. Patients like Jill Jones, who have conditions such as non-Hodgkin lymphoma, are already benefiting from these treatments. This initiative is part of the government's Plan for Change, which focuses on strengthening domestic medical supply chains and boosting economic growth.

- Total News Sources
- 1
- Left
- 0
- Center
- 1
- Right
- 0
- Unrated
- 0
- Last Updated
- 335 days ago
- Bias Distribution
- 100% Center
Open Story
Timeline
Analyze and predict the
development of events
Stay in the know
Get the latest news, exclusive insights, and curated content delivered straight to your inbox.

Gift Subscriptions
The perfect gift for understanding
news from all angles.














