Negative
24Serious
Neutral
Optimistic
Positive
- Total News Sources
- 4
- Left
- 3
- Center
- 1
- Right
- 0
- Unrated
- 0
- Last Updated
- 30 days ago
- Bias Distribution
- 75% Left
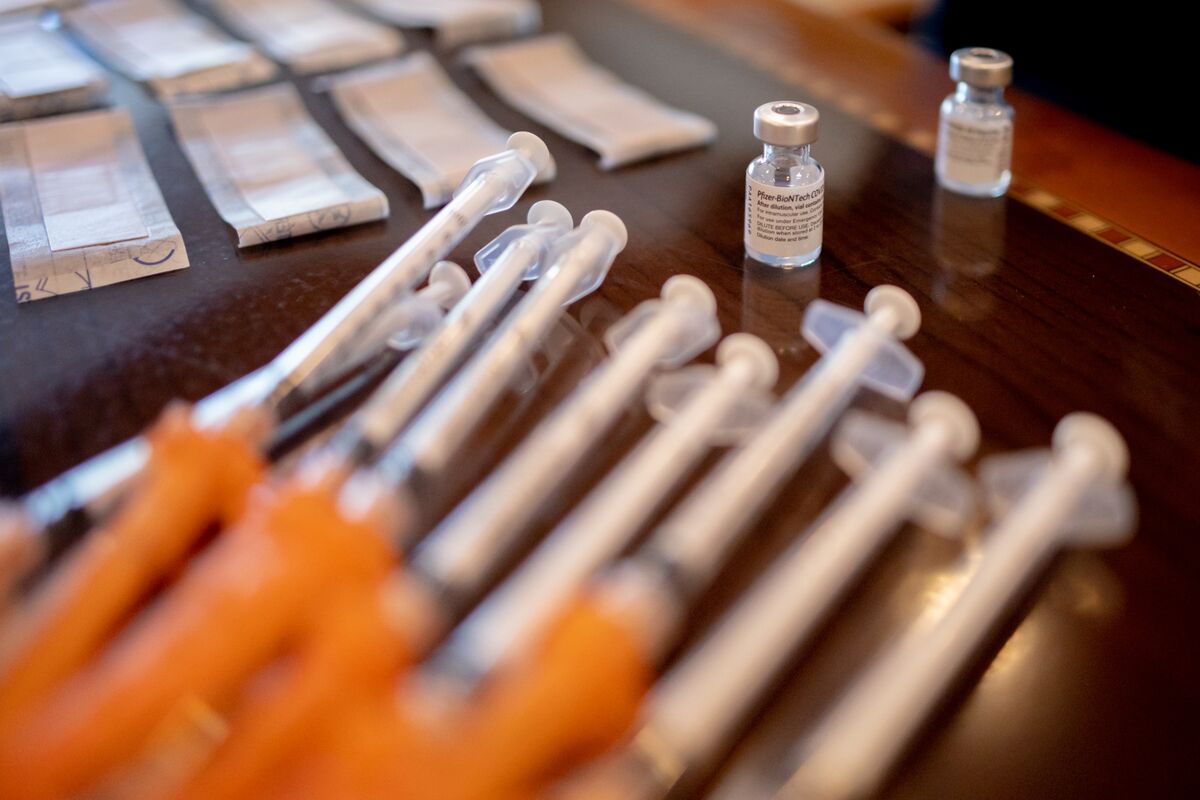

FDA Limits COVID-19 Shot Approval to Elderly, High-Risk Groups
The FDA has announced a significant shift in its COVID-19 vaccine approval process, narrowing eligibility to adults over 65 and individuals of any age with at least one high-risk condition, such as asthma, diabetes, or obesity. This new framework requires vaccine manufacturers to conduct randomized controlled trials for healthy individuals aged 6 months to 64 years before booster approval, aiming to generate robust evidence on vaccine safety and effectiveness for lower-risk populations. FDA officials, including Commissioner Marty Makary and vaccine division head Vinay Prasad, emphasized balancing timely access for high-risk groups with the need for rigorous data among healthier populations, potentially limiting broad vaccine availability. This approach aligns the U.S. with European risk-based vaccination strategies, moving away from the previous one-size-fits-all annual booster model. The FDA's advisory panel is set to recommend the 2025-2026 COVID-19 vaccine formula, potentially targeting the LP.8.1 subvariant, reflecting ongoing efforts to adapt vaccines to circulating strains. The policy change may reduce vaccine use among healthy children and adults unless new trials demonstrate continued vaccine benefits for these groups.



- Total News Sources
- 4
- Left
- 3
- Center
- 1
- Right
- 0
- Unrated
- 0
- Last Updated
- 30 days ago
- Bias Distribution
- 75% Left
Negative
24Serious
Neutral
Optimistic
Positive
Related Topics
Stay in the know
Get the latest news, exclusive insights, and curated content delivered straight to your inbox.

Gift Subscriptions
The perfect gift for understanding
news from all angles.


