Negative
24Serious
Neutral
Optimistic
Positive
- Total News Sources
- 1
- Left
- 1
- Center
- 0
- Right
- 0
- Unrated
- 0
- Last Updated
- 5 days ago
- Bias Distribution
- 100% Left
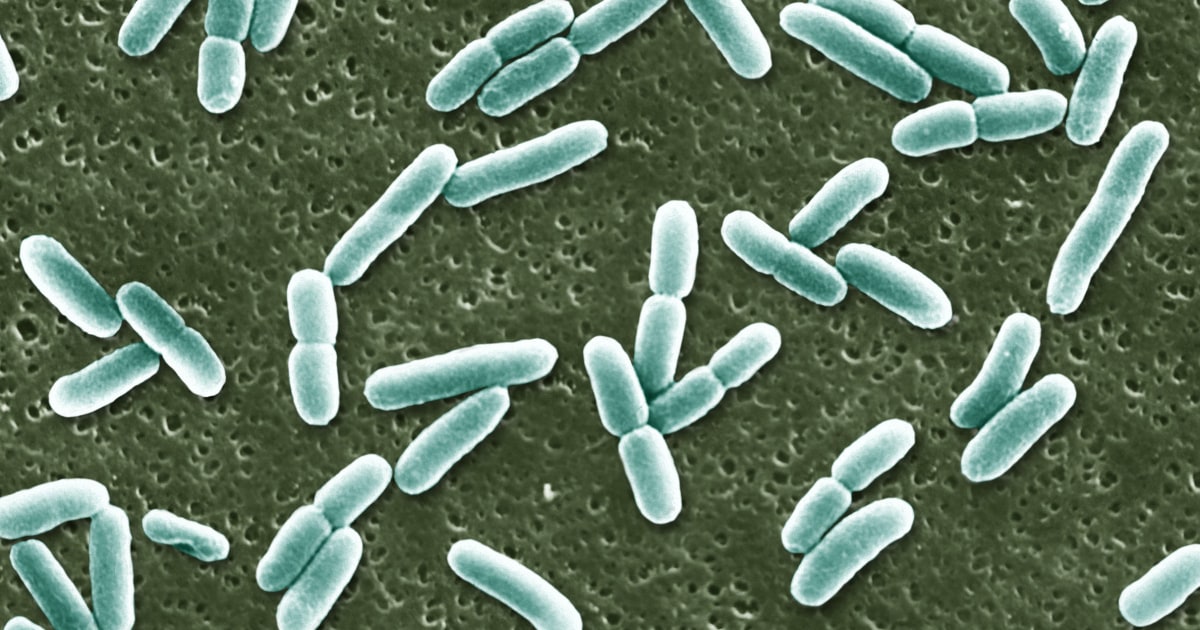

FDA Approves First New UTI Antibiotic in Nearly 30 Years
The FDA has approved Blujepa, a new oral antibiotic for treating uncomplicated urinary tract infections (UTIs), marking the first such approval in nearly 30 years. Developed by GSK, Blujepa is intended for women and girls aged 12 and older and has shown competitive efficacy in clinical trials compared to standard treatments like nitrofurantoin. With UTI cases rising, driven by increasing antibiotic resistance, the need for new treatment options has become critical; over 2.8 million antimicrobial-resistant infections occur annually in the U.S. Health experts emphasize the importance of developing antibiotics that target bacteria in novel ways to combat resistance. Blujepa is expected to be available in the latter half of the year, providing a much-needed alternative for patients struggling with recurrent infections. The approval reflects ongoing efforts in addressing the healthcare challenges posed by antibiotic-resistant bacteria.

- Total News Sources
- 1
- Left
- 1
- Center
- 0
- Right
- 0
- Unrated
- 0
- Last Updated
- 5 days ago
- Bias Distribution
- 100% Left
Negative
24Serious
Neutral
Optimistic
Positive
Related Topics
Stay in the know
Get the latest news, exclusive insights, and curated content delivered straight to your inbox.

Gift Subscriptions
The perfect gift for understanding
news from all angles.

